TTP developed CoVent™ for the UK Government in record time during the novel coronavirus pandemic.
Context
In March 2020, against the background of a predicted shortage of critical care ventilators for COVID-19 patients, we answered an urgent call for rapid manufacture ventilators from the UK Government.
Solution
As a multi-disciplinary technology company with significant experience in medical device development, we were able to tackle all aspects of the development of a new ventilator and created the technical file to support its rapid approval.
Result
In just 5 weeks following the call from the UK Government – much faster than the typical 3 to 5 years – CoVent was ready for regulatory approval and mass production. Out of over 5,000 offers of support, TTP was one of only five that were successful, and achieved this result at a lower cost than others.
As the COVID-19 pandemic threatened to overwhelm health services in many countries in March 2020, the UK Government asked a group of medical device development companies and manufacturers to step in and assist in the rapid provision of additional ventilators for COVID-19 patients.
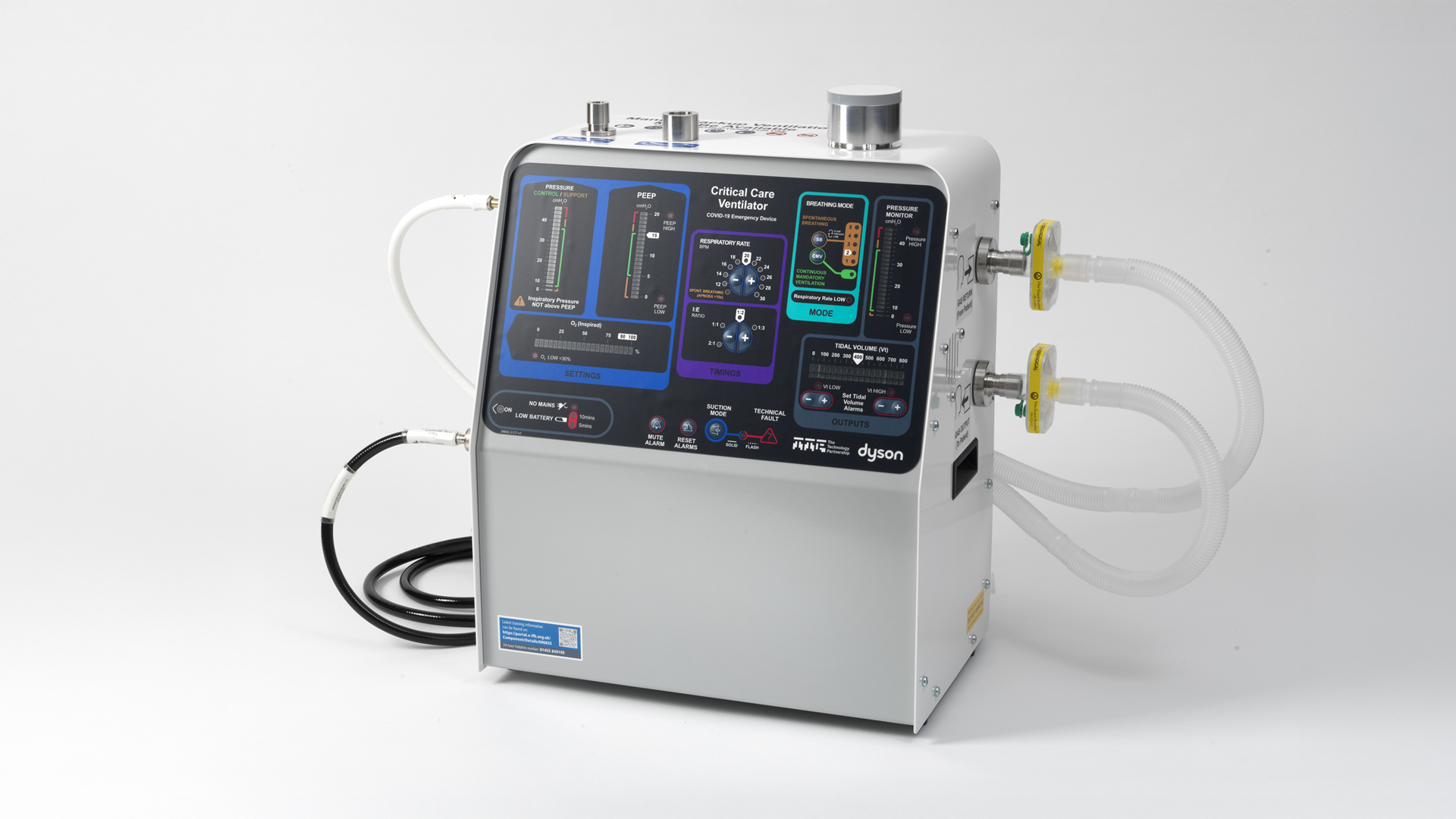
TTP was asked to develop a new, rapidly manufacturable ventilator using only parts readily available in the UK supply chain and that could be manufactured in volumes of thousands per week, while other companies in the UK Ventilator Challenge focused on ramping up the production of existing ventilator designs.
In addition, the Government specification called for a device that would be highly intuitive to use, including by clinicians who were not specialists in caring for patients that require ventilation.
The initial timeframe until the devices were needed for COVID-19 patients in the UK’s National Health Service was 6-8 weeks, an extraordinary challenge for medical device development.
Given the urgency of the impending national emergency, TTP focused the majority of its expertise and resources on the challenge and immediately began work on a clean-sheet design for a new ventilator.
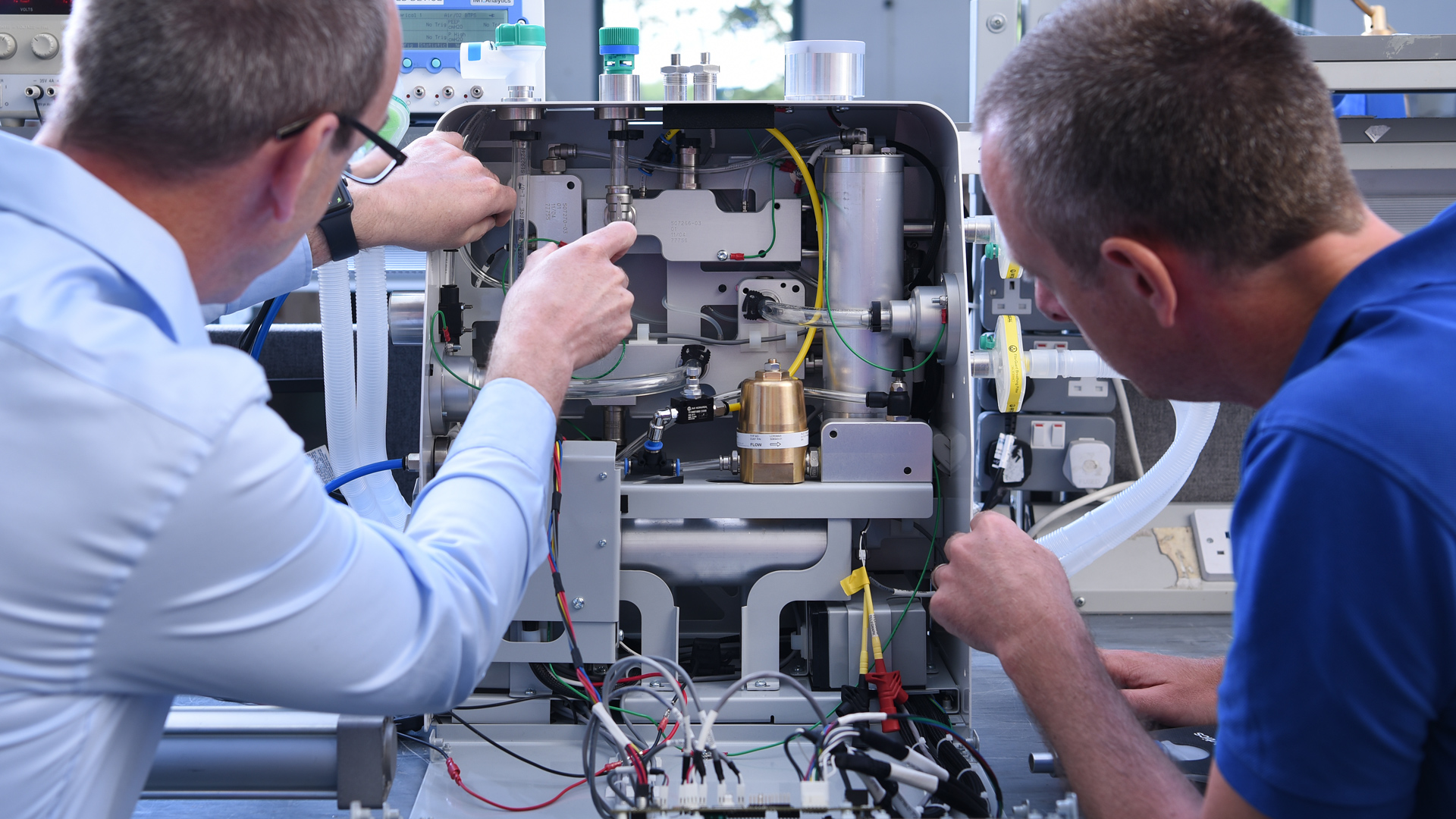
TTP teams tackled all aspects of the challenge in a highly parallel, around-the-clock development effort.
This included understanding clinical and user needs through early clinician input, development of the pneumatic air moving solution at the heart of CoVent, mechanical and electronic design of the device, as well as work on the user interface, which was anticipated to be of critical importance during clinical deployment.
Further teams established the supply chain and collaborated with manufacturing partner Dyson to set up production facilities for CoVent.
This approach to the development allowed us to rapidly achieve milestones and implement changes as the device specification evolved with growing understanding of the clinical needs of COVID-19 patients.
An overall design was selected, early prototypes assembled and their operation demonstrated within the first week.

CoVent’s user interface progressed equally fast. Early sketches rapidly evolved into the intuitive layout, which featured in news reports as the production of additional ventilators was keenly anticipated around the world at the height of the pandemic.
As the clinical picture of the disease changed, we rapidly implemented additional functions for suction to remove lung secretions and support for spontaneous breathing.
By the time the pandemic reached its peak in Europe and North America, the manufacturing line for CoVent at Dyson was ready for volume production, and the first production CoVent devices were submitted to UK regulators for approval.
Despite the rapid timeframe, the entire CoVent development was carried out in line with ISO 13485, as well as other relevant standards, to ensure the safety and effectiveness of the design and to support the rapid approval of the ventilator, with TTP acting as the certified design authority throughout.
But – thankfully – the anticipated national ventilator shortage was averted through social distancing and suppression of the virus. Whilst CoVent met all the specified requirements for COVID-19 patients, the approvals process for TTP’s submission for CoVent was therefore not taken forward by UK regulators.
TTP’s contribution to the UK Ventilator Challenge has been recognised by the UK Government.
The work to develop the CoVent device to this point has been extraordinary. It is a credit to TTP and your partners for accelerating a process which typically takes years to a number of weeks. I would like to offer my greatest appreciation and thanks for the quite extraordinary endeavour, enterprise and ingenuity shown by all involved in the CoVent ventilator.
Rt Hon Michael Gove MP
Chancellor of the Duchy of Lancaster and Minister for the Cabinet Office

Learn more about TTP's approach to medical device design and development and our medical device consulting services.